The use of antioxidants in animal husbandry is intended to counteract the deleterious consequences of free radicals from feed or metabolism on health and performance. Taking the usual precautions to reduce the risks (feed with less unsaturated lipids for example) will not be enough to totally avoid oxidative stress. Unsaturated fatty acids have virtues, farm animals have a very high metabolism and produce free radicals. Antioxidants will therefore have to be used.

This raises a question: why choose one antioxidant over another? We will not deal here with the strategy of feed protection as a complement to that of animals. The question that remains is that of comparing the effectiveness of one product rather than another in a given application.
Antioxidants: The different analytical methods for estimating effectiveness
One of the criteria put forward by feed additive manufacturers is the comparative efficacy of their product versus vitamin E. But each one has its own method. Although the ORAC method seems to be the reference, it is not always used in the same way.
2 main families of analytical methods available
All the methods for analysing the “antioxidant” power of a product are based on an in vitro oxidation-reduction reaction.
Example of a reaction, the oxidation of H2 by fluorine: H2 + F2 → 2 HF
It is always a question of directly or indirectly measuring the disappearance or appearance of one of the compounds in the reaction. There are 2 main families of classification of the antioxidant power of a product:
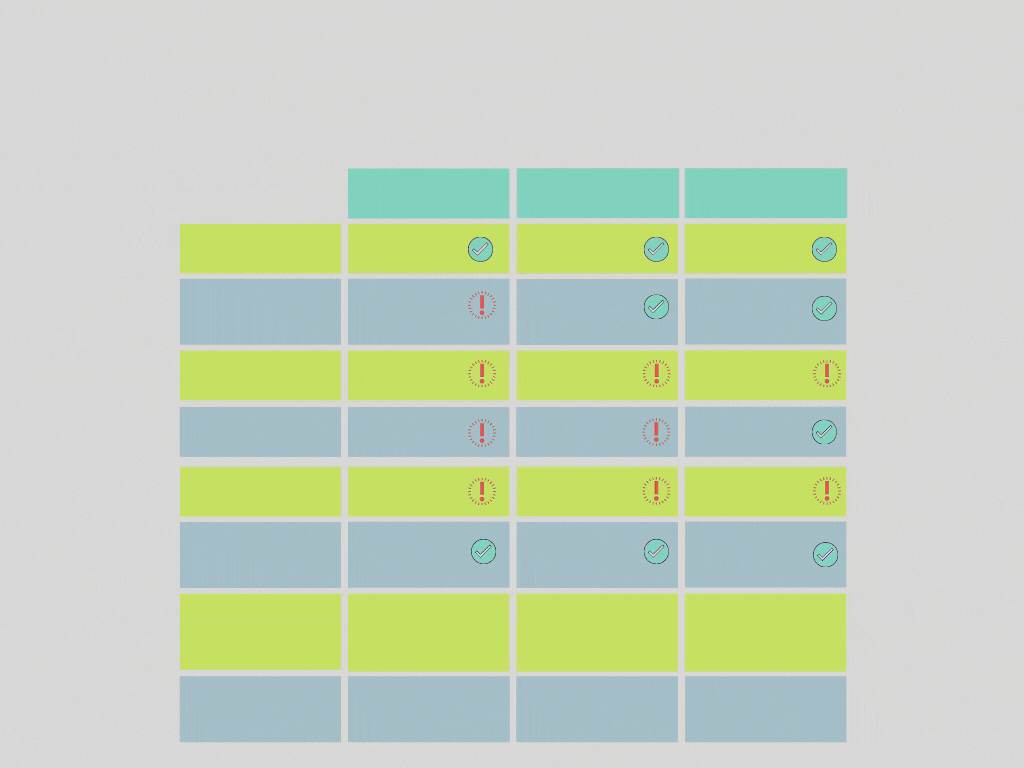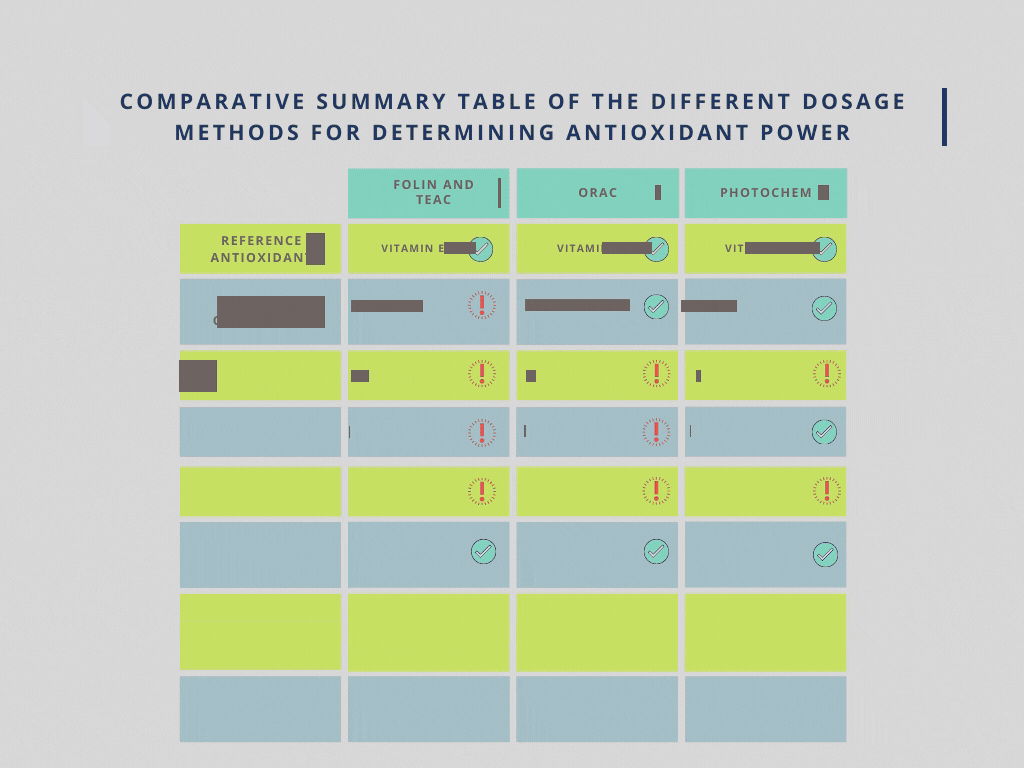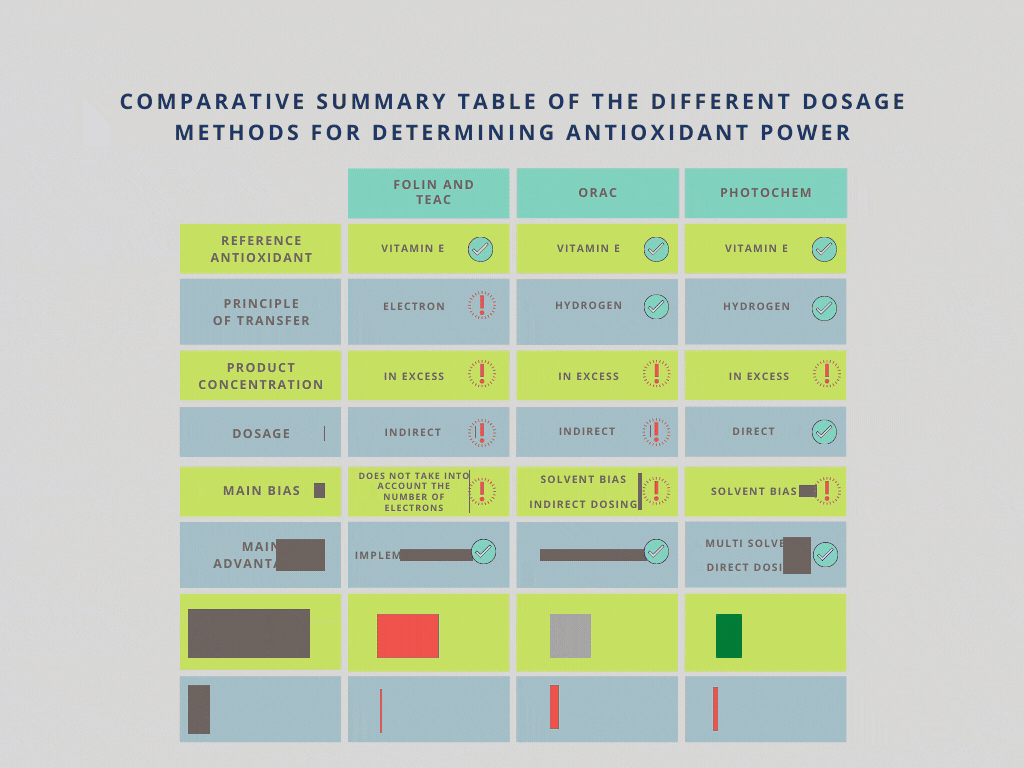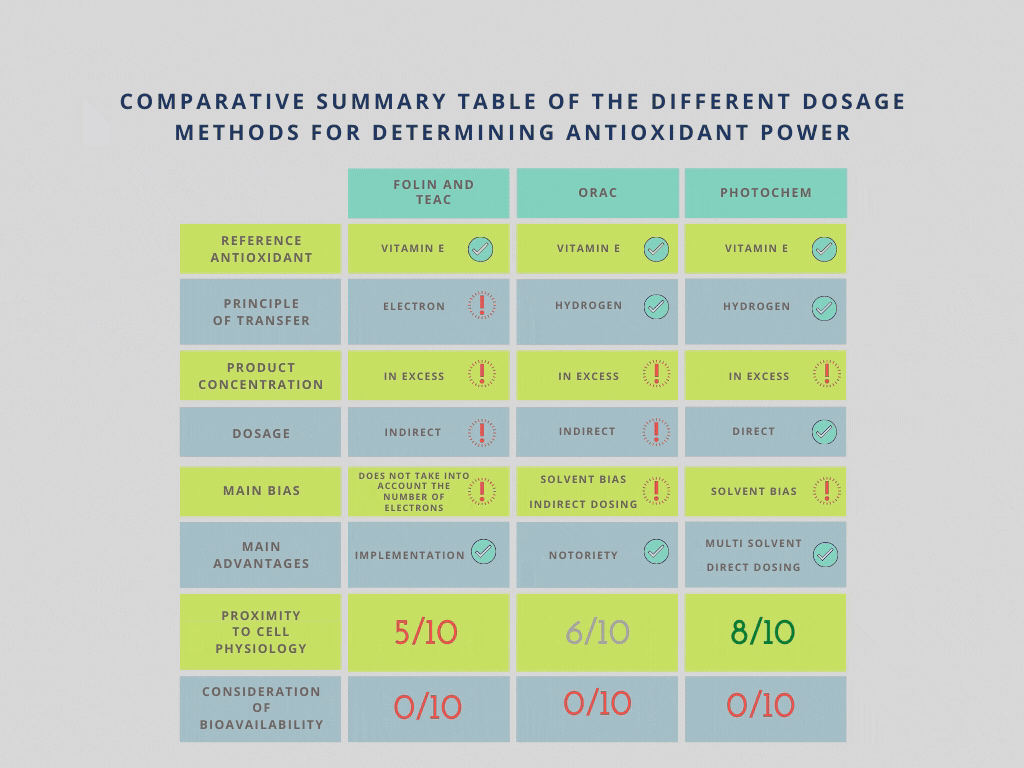
– methods based on electron transfer: Folin, TEAC
– methods based on H transfer: ORAC, Photochem
Which family can be selected?
Methods based on electron transfer do not take into account the number of electrons that antioxidants can provide. Their results are therefore unreliable when comparing 2 products. However, they are still useful for quality control of a given product in order to check its conformity with its specifications.
Methods based on hydrogen transfer are certainly more reliable but remain theoretical because the product tested is always in excess to ensure that the chemical reaction is as complete as possible. This situation is far from being representative of what happens in vivo.
The Photochem method uses the disappearance of a radical very present in the cells: O2-. The ORAC method uses an indirect dosage of a synthetic radical. This method is therefore less direct and less representative of the metabolism.
The importance of the solvents used
The ORAC method is the most recognised. However, there are many variations of this method, which are somewhat disruptive to the game. The product has to be solubilised, for which the ORAC method offers a variant for water-soluble products and a variant for fat-soluble products. For fat-soluble products, there are as many variants as there are suitable solvents. While TROLOX (water-soluble Vitamin E) is always the reference, the choice of solvent has a major influence on the result obtained.
The Photochem method is much less of a reference than the ORAC one. On the other hand, it is open to more types of solvents such as alcohols for example. However, although this method allows working with a wider spectrum of antioxidants, the solvent effect remains a bias and here too, all comparisons are prohibited.
Comparative summary table of the different dosage methods for determining the power of antioxidants
How to compare results obtained with different methods?
In order to be able to compare all these products on an equal footing, some authors propose to report everything in TROLOX equivalent or vitamin E equivalent. All these methods have in common that they compare the capacity of an antioxidant with that of TROLOX. This is absolutely not possible because the ratios between antioxidants and vitamin E change not only from one method to another, but also from one solvent to another. It is therefore illusory to compare them in absolute terms. These methods are not made for that.
Analytical methods to verify product conformity
On the other hand, these methods remain particularly useful for checking that a product complies with its specifications in relation to a given method. Indeed, antioxidants are by nature unstable and degrade over time. The determination of their expiry date and their quality must be followed by a strict control plan. In this case, the same protocol will always be used and the results will be comparable.
What about the mode of action of antioxidants in all this?
Moreover, if we need several different methods to measure the power of antioxidants of different products, it is also because their mode of action and the environments in which they will act are different. This is why it is better to look for complementarity, at the very least a targeting of the product on the animal’s problems. For example, prefer an antioxidant based on curcumin for a problem with inflammatory repercussions or rather grape procyanidins to reduce intestinal free radicals. Finally, only the animal can tell us what the effective dose is for a given problem.
Conclusion
All these in vitro methods have varying qualities but the same defects:
– The tested antioxidant is in excess for the good course of the redox reaction whereas in nature it is the opposite.
– None of these methods can take into account the bioavailability of an asset.
So the best method is to ask the animal what it thinks. In vivo tests with a blood parameter of oxidative status correlated with good production indicators will always remain the only justices of the peace.